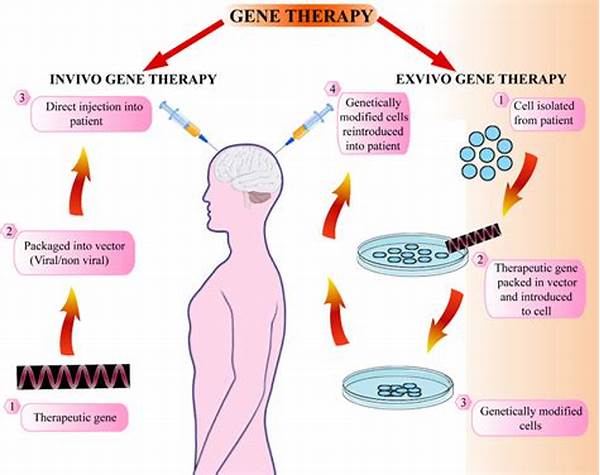
In recent years, gene therapy has emerged as a groundbreaking field with the potential to transform modern medicine by providing innovative treatments for genetic disorders. However, despite its vast potential, the path toward the successful implementation of gene therapy is fraught with numerous regulatory challenges. Navigating these intricacies requires a comprehensive understanding of the regulatory landscape, which governs the development, approval, and monitoring of gene-based treatments.
Read Now : Techniques For Addressing Adherence Barriers
The Complexity of Regulatory Frameworks
Gene therapy, by its very nature, demands rigorous oversight due to the profound impact it can have on human health. The regulatory challenges in gene therapy are primarily rooted in its novel approach to treatment, which involves manipulating genetic material to achieve therapeutic outcomes. Regulatory agencies worldwide, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established intricate frameworks for evaluating the safety and efficacy of gene therapy products. These frameworks necessitate extensive preclinical and clinical trials, stringent quality controls, and continuous post-market surveillance. The complexity of these regulatory processes often leads to prolonged approval timelines and significant costs. Furthermore, given the evolving landscape of gene therapy, regulatory pathways must continuously adapt to incorporate the latest scientific advancements and address emerging ethical considerations associated with genetic modifications.
Ensuring Patient Safety and Efficacy
Ensuring patient safety remains a paramount concern in the development of gene therapies. Regulatory challenges in gene therapy include identifying potential adverse effects and establishing long-term safety profiles. As gene therapies can lead to permanent genetic changes, any unintended consequences must be meticulously scrutinized. Additionally, demonstrating efficacy poses its own set of challenges, as traditional endpoints may not be applicable, necessitating the development of novel biomarkers and surrogate endpoints to assess therapeutic outcomes.
Ethical and Social Considerations
The regulatory challenges in gene therapy extend beyond scientific and technical aspects, encompassing ethical and social dimensions. The potential for germline modifications raises profound ethical questions regarding gene editing and its implications for future generations. Regulatory bodies must weigh these societal concerns while ensuring that the benefits of gene therapy are accessible to those in need. Furthermore, balancing innovation with caution is crucial in preventing misuse or over-commercialization of gene-based interventions.
Slang Perspective on Regulatory Challenges
1. Gene therapy ain’t just a walk in the park, man. The regulatory challenges in gene therapy are a real uphill battle, with heaps of red tape to cut through.
2. Trust me, dealing with the regulatory challenges in gene therapy is like trying to solve a Rubik’s cube blindfolded—there’s just no easy way around it.
3. Regulators wanna make sure gene therapy is the real deal, no shortcuts allowed—these regulatory challenges are no joke!
4. Prepping for gene therapy trials? Buckle up, ’cause the regulatory challenges in gene therapy will put you through the wringer.
Read Now : Novel Cell Therapy Breakthroughs
5. Yo, when it comes to gene therapy, navigating those regulatory hurdles is like threading a needle in a haystack, seriously tricky stuff.
Advancements in Regulatory Science
The regulatory challenges in gene therapy necessitate advancements in regulatory science to keep pace with rapid technological developments. Regulators are increasingly incorporating adaptive frameworks and expedited pathways to facilitate timely approval of innovative therapies. Collaborative efforts among global regulatory bodies and stakeholders aim to harmonize guidelines, ensuring consistent evaluation criteria across jurisdictions. Furthermore, the integration of real-world evidence and advanced analytics is revolutionizing regulatory decision-making processes, providing valuable insights into the long-term safety and effectiveness of gene therapies.
Bridging the Gap Between Innovation and Regulation
Efforts to address regulatory challenges in gene therapy are focused on fostering a balance between innovation and rigorous oversight. Regulatory agencies are engaging with scientific communities and industry stakeholders to develop comprehensive guidance documents and establish collaborative forums. By encouraging transparency and open dialogue, regulators aim to build trust and facilitate the development of robust regulatory strategies that accommodate cutting-edge advancements. This collaborative approach is essential for ensuring that gene therapies reach patients safely and efficiently, while maintaining public confidence in the regulatory system.
Conclusion
In conclusion, while gene therapy holds immense promise for revolutionizing healthcare, the regulatory challenges it presents are complex and multifaceted. Addressing these challenges requires a concerted effort from regulatory bodies, industry stakeholders, and the scientific community. By fostering collaboration and adopting adaptive regulatory frameworks, we can navigate the intricacies of gene therapy regulation, ultimately paving the way for innovative treatments that enhance patient outcomes. The continued evolution of regulatory science is essential to unlocking the full potential of gene therapy, ensuring that this transformative field benefits patients worldwide.