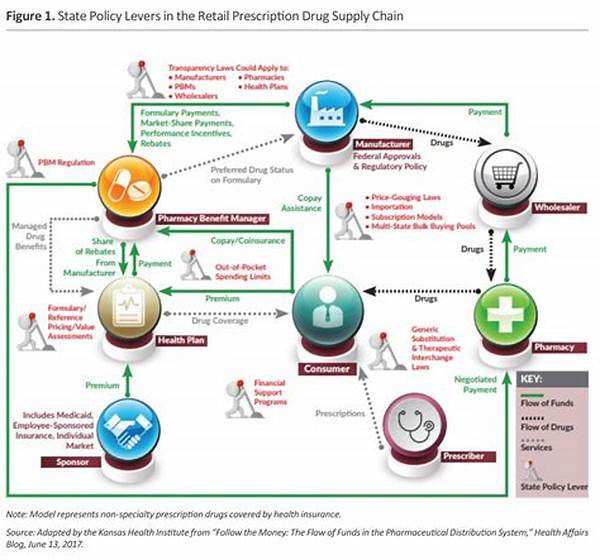
The Importance of Prescription Drug Access Regulations
Prescription drug access regulations play a pivotal role in ensuring that medications are both available and affordable to those who need them. These regulations bridge the gap between pharmaceutical innovations and public health needs. Their primary objective is to guarantee that safe and effective drugs are accessible to the population, which necessitates a delicate balance between controlling costs and incentivizing innovation.
Read Now : “best Prescription For Controlling High Blood Pressure”
One of the key factors in prescription drug access regulations is the approval process. Regulatory agencies, such as the FDA in the United States, evaluate the safety and efficacy of medications before they reach the market. This ensures that the drugs available to consumers have been rigorously tested and are deemed safe for use. Additionally, these regulations may include pricing controls and guidelines to prevent excessive pricing that could deter access for those in need.
Moreover, prescription drug access regulations encompass measures to prevent monopolistic practices in the pharmaceutical industry. By fostering a competitive market, these regulations aim to enhance accessibility while encouraging continuous advancements in drug development. They often involve policies related to patents, generic drug manufacturing, and market exclusivity, which collectively contribute to making medications accessible to a broader population.
Key Components of Prescription Drug Access Regulations
1. Approval Process: Prescription drug access regulations ensure that drugs undergo stringent testing for safety and efficacy. This process guarantees that only effective medications are approved for public use.
2. Pricing Controls: Regulations help maintain affordable prices by implementing measures to control drug costs. This prevents exorbitant pricing and enhances accessibility for all.
3. Market Competition: Encouraging competition through regulations limits monopolistic practices, thus broadening prescription drug access for diverse populations.
4. Generic Drug Availability: Prescription drug access regulations support the production of generic medications post-patent expiration, increasing accessibility and reducing costs.
5. Insurance Coverage: These regulations often dictate insurance policies to include comprehensive drug coverage, ensuring patients can afford prescribed treatments.
Challenges in Implementing Prescription Drug Access Regulations
Despite their importance, the implementation of prescription drug access regulations faces numerous challenges. Rising drug costs have become a focal point, necessitating stricter regulatory oversight to ensure fairness and transparency. Balancing cost control with the promotion of innovation poses a complex dilemma for policymakers.
Furthermore, disparities in global health systems contribute to uneven access across different regions. Developed nations might enjoy a more streamlined process of drug availability, while developing countries face hurdles due to resource limitations and regulatory discrepancies. Harmonizing international prescription drug access regulations to ensure equitable access remains a pressing issue on the global health agenda.
Additionally, regulatory authorities must constantly adapt to the rapid advancements in biotechnology and personalized medicine. The dynamic nature of the pharmaceutical landscape requires agile and responsive strategies to accommodate novel therapies without compromising safety and affordability. Hence, continuous evaluation and reform are imperative for the effective implementation of prescription drug access regulations.
Prescription Drug Access Regulations: A Casual Perspective
Prescription drug access regulations are, like, super important, you know? They make sure meds don’t cost an arm and a leg. Plus, they keep big pharma in check, so we don’t get ripped off. These rules are all about making sure everyone gets their hands on the drugs they need without breaking the bank.
1. Regulations stop drugs from being crazy expensive.
2. They make sure meds are safe and do their job.
3. Help bring cheaper generic drugs to the market.
4. Prevent big companies from taking over the drug scene.
5. Ensure health insurance covers necessary meds.
Read Now : Safe Combination Of Herbs And Drugs
6. Strive for fair drug pricing worldwide.
7. Push for innovation while controlling costs.
8. Adapt to new medical breakthroughs.
9. Help patients get affordable treatment.
10. Keep the drug market competitive and fair.
The Role of Government in Prescription Drug Access Regulations
Government agencies play a crucial role in shaping prescription drug access regulations. By enacting laws and policies, they establish a framework that governs the production, distribution, and pricing of pharmaceuticals. This regulatory framework aims to ensure that medications remain safe, effective, and affordable for the general public.
Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States, are tasked with the evaluation and approval of new drugs. They conduct meticulous reviews of clinical trial data to verify the safety and efficacy of medications before granting them market access. This process is critical in safeguarding public health and preventing the distribution of untested or harmful drugs.
In addition to approval processes, government policies often include provisions for price controls and market competition. These regulations are designed to prevent exploitative pricing practices and to encourage the availability of cost-effective alternatives, such as generic medications. Such measures are essential in ensuring that prescription drugs are accessible to individuals from all socioeconomic backgrounds.
International Collaboration for Prescription Drug Access Regulations
In an increasingly globalized world, international collaboration has become paramount in establishing effective prescription drug access regulations. Harmonizing standards and practices across different countries facilitates the availability of medications and streamlines regulatory processes. Collaborative efforts among international health organizations help in sharing information, resources, and expertise to address global challenges in drug accessibility.
One of the key benefits of international collaboration is the ability to pool resources for research and development. Collaborative initiatives enable countries to work together in creating innovative solutions to complex health issues. Additionally, these partnerships can assist in the equitable distribution of medications, ensuring that even low-resource nations have access to vital drugs.
Moreover, leveraging global data and knowledge can enhance the adaptability and responsiveness of prescription drug access regulations. By sharing insights on emerging health trends and drug efficacy, nations can collectively improve their regulatory frameworks. Continued international cooperation is essential in addressing the multifaceted challenges of drug accessibility on a global scale.
Conclusion on Prescription Drug Access Regulations
In conclusion, prescription drug access regulations are integral in maintaining the delicate balance between innovation in pharmaceuticals and public health needs. Through stringent approval processes and policies promoting market competition, these regulations aim to ensure that medications are safe, effective, and accessible to all individuals.
Although challenges persist, ongoing efforts to address rising drug costs and international disparities in access demonstrate the commitment to refining and improving these regulations. International collaboration plays a pivotal role in harmonizing standards, facilitating information sharing, and fostering innovations that benefit global health.
Ultimately, the success of prescription drug access regulations hinges on their ability to adapt to technological advancements while ensuring equitable access. Continuous evaluation and reform are necessary to meet the evolving needs of society and to uphold the fundamental principles of health equity and justice.