The significance of biomarker validation processes cannot be overstated in contemporary scientific and clinical research. Biomarkers serve as critical indicators of biological states, providing valuable insights into the diagnosis, prognosis, and monitoring of diseases. Ensuring their reliability and accuracy through rigorous validation processes is imperative to achieving advancements in medical science. This comprehensive article elucidates various aspects of biomarker validation, underscoring its pivotal role in the transition from research to clinical application.
Read Now : Genomics-based Customized Healthcare
The Importance of Biomarker Validation
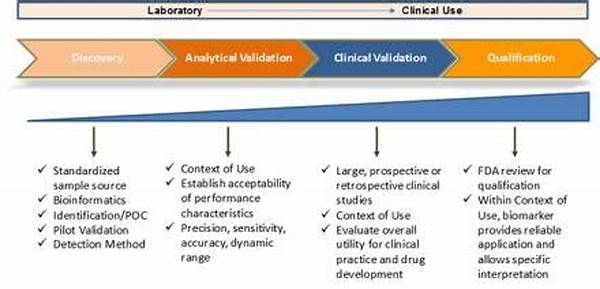
Biomarker validation processes are essential for the translation of promising biomarkers from the laboratory to real-world clinical settings. This meticulous procedure involves a series of stages that aim to affirm the reproducibility, sensitivity, and specificity of the biomarker in question. Initially, exploratory studies assess the potential utility of a biomarker. Subsequently, a validation phase is conducted, wherein larger and more diverse populations are examined to ascertain generalizability. The ultimate goal is to provide reliable evidence that supports the biomarker’s utility in diagnosing or prognosticating a specific condition. These validation processes not only enhance the credibility of scientific findings but also foster the development of precision medicine. By integrating validated biomarkers into clinical practice, healthcare providers can tailor interventions to individual needs, thereby improving patient outcomes.
Challenges in Biomarker Validation
1. Complexity of Biological Systems: Biomarker validation processes must accommodate the inherent complexity of biological systems, which often exhibit significant variability among individuals. This complexity demands robust study designs to ensure the accuracy of results.
2. Data Integration and Management: High volumes of data are generated during biomarker validation processes, necessitating effective integration and management strategies. This requires sophisticated analytical tools and methodologies.
3. Regulatory Compliance: Biomarker validation processes are subject to stringent regulatory requirements, ensuring that biomarkers meet established standards before clinical implementation.
4. Resource Intensity: Conducting biomarker validation processes is resource-intensive, often requiring substantial financial and temporal investments to achieve conclusive results.
5. Translational Gaps: Bridging the gap between discovery and clinical application is a common hurdle in biomarker validation processes, demanding close collaboration between researchers and clinicians.
Methodologies in Biomarker Validation
Biomarker validation processes encompass a myriad of methodologies tailored to ensure a biomarker’s efficacy and reliability. High-throughput technologies, such as genomics and proteomics, play a pivotal role in these processes, facilitating comprehensive analysis of potential biomarkers. Validation requires a multi-phase approach, starting with analytical validation, which assesses a biomarker’s measurement accuracy. Clinical validation follows, confirming the biomarker’s predictive capabilities within a specific clinical context. Finally, clinical utility validation examines the impact of the biomarker on patient management and outcomes. Such meticulous methodologies are indispensable to overcoming the intricacies associated with biomarker validation. By adhering to strict validation protocols, we can ensure that only biomarkers with proven efficacy are integrated into clinical practice, thus reinforcing the integrity of precision medicine.
Understanding Biomarker Validation: A Slang Perspective
Okay, so here’s the lowdown on biomarker validation processes. Consider it the crucial step in ensuring that the cool new biomarker you found in the lab isn’t just a one-hit-wonder. It’s about taking it from the bench to the bedside, making sure it works in the real world and isn’t just science fiction.
1. Hardcore Data Checks: You need to crunch loads of data to make sure your biomarker actually does what it claims. No room for errors here.
2. Lab Rats to Real Humans: Yeah, it’s great that it worked on mice; now let’s see if it works just as well on actual sick people.
3. Number Games: Statistical shenanigans to determine if that biomarker can handle the pressure of real diseases.
4. Gold Standard Goals: Before going big, it has to meet those top-notch medical standards. Cure-all or bust, right?
Read Now : Custom Treatment Plans Using Genomic Insights
5. Cash and Time Drains: Be ready to pour in money and months, because good science ain’t cheap!
6. Red Tape Rumble: Navigating through enough legal hoops to make your head spin.
7. Data Dilemmas: Finding badass ways to organize and interpret that massive data pile.
8. Crossing Bridges: Collaborate with other smart folks to make it clinically mainstream.
9. Trial While It’s Hot: Real-world human trials are where the magic — or reality check — happens.
10. Goal?: Get unique patient-centric treatments spinning off the shelves of clinic land.
Key Benefits of Biomarker Validation
Biomarker validation processes yield numerous advantages essential for enhancing clinical outcomes and advancing scientific understanding. Firstly, these processes ensure the reliability and reproducibility of biomarkers, fostering trust among healthcare professionals and patients. By rigorously verifying biomarker accuracy, clinicians can make informed decisions regarding diagnosis and treatment plans. Furthermore, biomarker validation processes pave the way for personalized medicine, where interventions are tailored based on individual biomarker profiles. This personalized approach holds significant potential to increase treatment efficacy while minimizing adverse reactions, thereby improving patient well-being. Additionally, validated biomarkers contribute to the identification of novel therapeutic targets, catalyzing the development of innovative drugs and treatment strategies. Consequently, validation processes play a pivotal role in narrowing the gap between research and clinical practice, facilitating the translation of scientific discoveries into life-saving interventions.
The Future of Biomarker Validation
As medical research progresses, the landscape of biomarker validation processes is poised for evolution. Emerging technologies such as artificial intelligence and machine learning offer unprecedented opportunities to enhance validation efficiency and accuracy. These technologies enable the analysis of vast data sets, elucidating complex biological mechanisms and bolstering the predictive capabilities of biomarkers. Furthermore, collaborative efforts across interdisciplinary fields, including genomics, bioinformatics, and clinical research, hold promise in overcoming existing challenges in validation processes. Integrating patient-centric approaches into biomarker validation is also gaining traction, ensuring that personalized medicine remains at the forefront of healthcare. Ultimately, the dedicated pursuit of innovative biomarker validation processes will continue to revolutionize medical research, facilitating the transition toward precision medicine that is both effective and accessible.
Conclusion: Navigating the Path Forward
In summary, biomarker validation processes are critical to the successful integration of biomarkers into clinical practice. These processes provide a comprehensive framework for evaluating biomarker reliability, guiding the development of personalized treatment strategies, and enabling groundbreaking advancements in medical science. Despite existing challenges, the future of biomarker validation appears promising, driven by technological innovations and collaborative efforts. By continually refining validation methodologies, the scientific community can ensure that biomarkers transcend from promising laboratory findings to impactful clinical tools. As the frontier of medical research expands, biomarker validation processes remain at the core of realizing the potential of precision medicine, offering hope for improved patient outcomes and a more profound understanding of complex diseases.