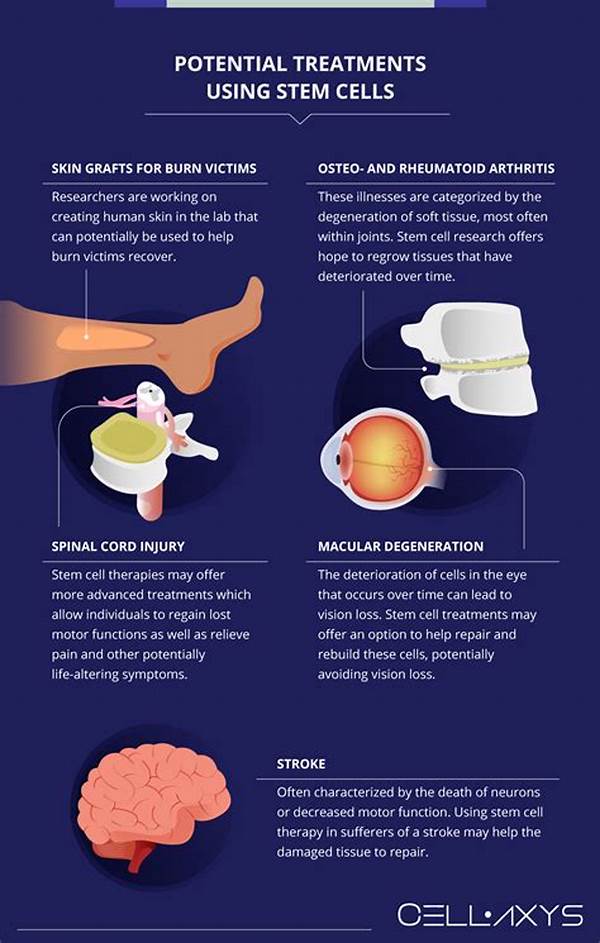
Advances in Stem Cell Research
Clinical trials in stem cell treatments have emerged as a promising frontier in medical research, aimed at harnessing the regenerative capabilities of stem cells to address various diseases and conditions. These trials represent a crucial step in translating basic scientific discoveries into practical therapeutic applications. Through rigorous testing and methodology, clinical trials aim to assess the safety, efficacy, and potential long-term effects of stem cell-based therapies. By meticulously analyzing outcomes, researchers seek to establish robust evidence for the clinical benefits of stem cells. Importantly, these trials also play a pivotal role in understanding the challenges and limitations inherent in stem cell therapies, thereby guiding future innovations in this rapidly evolving field. As the scientific community continues to make strides, the successful execution of these trials could significantly impact the treatment landscape for debilitating conditions, offering hope to patients worldwide. Consequently, clinical trials in stem cell treatments not only highlight the advancements in biotechnology but also embody a critical component of contemporary medical research dedicated to improving human health.
Read Now : **dna Methylation In Developmental Processes**
Ethical Considerations in Clinical Trials
1. Ethical considerations in clinical trials in stem cell treatments are paramount, as they ensure the protection of participants and guide responsible research practices.
2. The informed consent process in clinical trials in stem cell treatments is a key element, ensuring participants fully understand the procedures, potential risks, and benefits involved.
3. Regulatory oversight in clinical trials in stem cell treatments serves to maintain scientific integrity and compliance with ethical standards, ensuring research transparency.
4. Patient selection criteria in clinical trials in stem cell treatments are designed to identify suitable candidates, emphasizing safety and maximizing therapeutic outcomes.
5. Monitoring and reporting procedures in clinical trials in stem cell treatments are vital for tracking progress and adverse effects, ensuring the trial’s ethical and scientific validity.
Regulatory Frameworks and Challenges
Clinical trials in stem cell treatments must navigate a complex regulatory landscape, as they are governed by stringent guidelines designed to ensure both participants’ safety and scientific validity. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established comprehensive frameworks that researchers must adhere to, ranging from preclinical studies to post-market surveillance. These frameworks demand thorough documentation and validation of research methodologies to corroborate the trials’ integrity. Regulatory challenges in clinical trials in stem cell treatments often involve balancing the need for innovative therapies with the rigorous safety standards essential for patient protection. Moreover, the dynamic nature of stem cell research necessitates continual updates and adaptations in regulatory measures to accommodate scientific advancements. Addressing these challenges is essential for facilitating the translation of groundbreaking stem cell research into viable clinical interventions. As the field progresses, improving regulatory understanding and resolving logistical hurdles will be crucial for advancing stem cell therapies from the laboratory to clinical practice, ultimately benefiting patients with conditions unresponsive to conventional treatments.
Slang Overview of Clinical Trials
1. Clinical trials in stem cell treatments are like the hottest gig in the science world right now—everyone’s buzzing about it.
2. When it’s about cool medical stuff, clinical trials in stem cell treatments top the charts.
3. Diving deep into clinical trials in stem cell treatments feels like decoding the future of healthcare.
4. Clinical trials in stem cell treatments? That’s the real VIP pass in medical research today.
5. Picture clinical trials in stem cell treatments as the trending topic that everyone wants a piece of.
Read Now : Implementing Green Initiatives In Pharmacies
6. In layman’s terms, clinical trials in stem cell treatments are science’s way of going big or going home.
7. Think of clinical trials in stem cell treatments as the ultimate remix of biology and medicine.
8. If you’re all about breakthroughs, clinical trials in stem cell treatments are where the action’s at.
9. Clinical trials in stem cell treatments are kind of like the viral sensation of medical studies.
10. Catching up with clinical trials in stem cell treatments is like being in-the-know with the A-listers of science.
Methodologies in Stem Cell Research
Clinical trials in stem cell treatments employ a range of sophisticated methodologies to ensure rigor and accuracy in the research process. These methodologies are meticulously designed to assess the therapeutic potential of stem cells across various conditions, encompassing a spectrum of scientific techniques. Randomized controlled trials, often considered the gold standard, are frequently utilized to eliminate bias and validate the efficacy of stem cell interventions. Additionally, adaptive trial designs are leveraged to allow for modifications in response to emerging data, enhancing the flexibility and responsiveness of the research. Double-blind studies are another critical component, ensuring that neither the participants nor the researchers know who is receiving the treatment versus a placebo, thus preserving objectivity. The incorporation of standardized protocols and outcome measures further fortifies the reliability of findings in clinical trials. Furthermore, the use of biomarker analysis aids in comprehensively understanding the biological mechanisms underpinning stem cell therapies, providing crucial insights into their function and impact. As the field advances, the refinement of these methodologies remains a priority, facilitating the generation of robust evidence that supports the clinical application of stem cell treatments.
Global Impact of Stem Cell Trials
The global impact of clinical trials in stem cell treatments is profound, heralding a new era in personalized medicine and offering hope for patients with conditions previously deemed intractable. These trials signify an important evolution in medical research, allowing scientists and healthcare providers worldwide to explore novel treatment modalities tailored to individual patient needs. As clinical trials progress, they contribute invaluable data to international registries, fostering a collaborative environment where cross-border scientific dialogues and shared knowledge thrive. This international collaboration underscores the potential of stem cell treatments to transcend geographical boundaries, ensuring that advancements benefit a broader spectrum of patients. Furthermore, the successful implementation of clinical trials in stem cell treatments could catalyze a paradigm shift in healthcare systems, emphasizing preventative medicine and regenerative therapies. Ultimately, as the world grapples with aging populations and the burden of chronic diseases, the insights and innovations arising from these trials hold the promise of transforming medical practice and improving health outcomes on a global scale.
Summary of Current Trends
In summary, clinical trials in stem cell treatments represent a dynamic and rapidly evolving area of biomedical research. Their primary objective is to rigorously evaluate the safety and effectiveness of stem cell therapies for a wide range of medical conditions. These trials encompass various phases, beginning with small-scale studies assessing safety and progressing to large-scale trials that evaluate therapeutic efficacy. The iterative nature of this research allows for continuous refinement and optimization of treatment protocols. As clinical trials in stem cell treatments advance, they are increasingly characterized by interdisciplinary collaboration, integrating expertise from fields such as genetics, immunology, and bioengineering. These collaborations are essential for addressing complex scientific questions and harnessing the full potential of stem cells in regenerative medicine. Furthermore, advancements in technology, such as gene editing and personalized medicine approaches, are enhancing the precision and targeting of stem cell therapies. Despite the challenges and regulatory hurdles that remain, the prospects for clinical trials in stem cell treatments are promising, offering a glimpse into a future where innovative therapies can significantly improve patient care and quality of life.