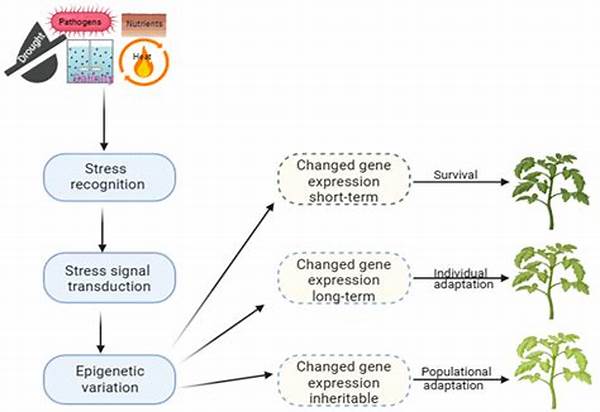
In recent years, the burgeoning field of epigenetics has revealed pivotal insights into how stress impacts human biology. Epigenetic mechanisms in stress response elucidate the intricate interplay between genetic predispositions and environmental factors. These mechanisms encompass a panoply of molecular modifications that do not alter the DNA sequence but significantly affect gene expression. Importantly, such modifications serve as mediators that translate external stressors into internal biological responses, thereby influencing an individual’s resilience to stress and susceptibility to stress-related disorders. As the prevalence of stress-related conditions escalates, understanding these mechanisms offers potential pathways for novel therapeutic interventions.
Read Now : Herbal Medicine Therapeutic Applications
The Role of DNA Methylation
DNA methylation is a crucial component of epigenetic mechanisms in stress response. In this process, methyl groups are added to DNA molecules, typically suppressing gene expression. During stress, certain genes may undergo hypermethylation, diminishing their activity, while others may be hypomethylated, leading to increased expression. This dynamic modulation can influence various physiological responses, such as the regulation of the hypothalamic-pituitary-adrenal axis and inflammatory processes. By manipulating these methylation patterns, therapeutic strategies can potentially mitigate the detrimental impacts of stress, underscoring the clinical relevance of epigenetic research. Through advanced scientific endeavors, researchers aim to illuminate the specific methylation targets involved in stress response, providing a clearer understanding of how genetic and environmental interplays shape health outcomes.
Histone Modification in Stress Adaptation
1. Histone modifications are integral to epigenetic mechanisms in stress response, altering chromatin structure and gene accessibility. These chemical changes influence how tightly DNA is wrapped around histones, thereby modulating gene expression.
2. Acetylation of histones typically enhances gene transcription, facilitating stress adaptation by activating stress-response genes. This process underscores the complexity of epigenetic regulation in maintaining homeostasis under duress.
3. Conversely, deacetylation is associated with gene repression, contributing to the suppression of unnecessary or harmful gene expression during prolonged stress. Such modulation is vital for resource allocation during stress periods.
4. Phosphorylation and methylation of histones further diversify the epigenetic landscape, enabling fine-tuned responses to various stressors. These modifications represent additional layers in the regulation of gene expression.
5. By employing inhibitors of specific histone-modifying enzymes, researchers can potentially restore aberrant gene expression linked to stress-induced pathologies. This insight highlights the therapeutic potential inherent in understanding histone-related epigenetic mechanisms in stress response.
MicroRNAs and Stress Regulation
MicroRNAs (miRNAs) represent a pivotal element in epigenetic mechanisms in stress response. These small, non-coding RNA molecules regulate gene expression post-transcriptionally and play a critical role in orchestrating cellular reactions to stress. By binding to target messenger RNAs, miRNAs can fine-tune protein synthesis and thereby impact diverse physiological processes. Under stress conditions, specific miRNAs are expressed or silenced, modulating cellular pathways involved in inflammation, metabolism, and even neural communication. The epigenetic regulation mediated by miRNAs underscores a sophisticated control system, aligning cellular function with environmental changes. Research has increasingly focused on identifying stress-responsive miRNAs and elucidating their specific roles, offering potential biomarkers for stress-related conditions and therapeutic targets. Insights into miRNA dynamics enhance our understanding of how organisms adapt to stress and maintain equilibrium.
Read Now : Dna Testing In Clinical Settings
The Future of Epigenetic Research in Stress
Understanding the full scope of epigenetic mechanisms in stress response remains an imperative frontier. The continually evolving landscape of research promises to yield breakthroughs in both the identification and manipulation of epigenetic factors associated with stress. Researchers endeavor to map the intricate network of modifications that dictate stress resilience, seeking to translate these findings into clinical applications. Embracing cutting-edge technologies, such as CRISPR-based editing and high-throughput sequencing, scientists aim to dissect the role of individual epigenetic marks in stress adaptation. By elucidating the precise epigenetic landscapes associated with stress and its physiological sequelae, an entirely new class of interventions targeting epigenetic modifications becomes conceivable.
Implications of Epigenetic Changes Under Stress
The implications of epigenetic mechanisms in stress response are profound and far-reaching. These molecular modifications offer explanations for individual variability in stress resilience and disorders, highlighting genetic regulation’s subtlety in health and disease. Understanding how life experiences, through stress or adversity, precipitate lasting epigenetic changes has revolutionized our view of gene-environment interactions. By exploring how these epigenetic modifications contribute to, or mitigate, stress-induced pathologies such as depression, anxiety, and PTSD, a new era in personalized medicine is on the horizon.
Epigenetic Interventions in Stress-Related Disorders
Epigenetic interventions hold promise in treating stress-related disorders. By targeting specific epigenetic modifications, it is possible to reverse maladaptive gene expressions. Drugs that modulate DNA methylation, histone modifications, or miRNA pathways represent burgeoning areas of research. Through precise interventions, these epigenetic therapies could ameliorate symptoms by restoring balanced physiological responses to stressors. The development of these therapeutics is bolstered by continual advancements in our understanding of the molecular underpinnings of stress, setting the stage for more effective strategies in managing stress-related disorders.
Summary of Epigenetic Mechanisms in Stress Response
In summary, epigenetic mechanisms in stress response illuminate the intricate interplay between an organism’s genome and its environment, facilitating appropriate physiological adjustments to external stressors. The integration of DNA methylation, histone modification, and miRNA regulation forms a complex regulatory network governing stress adaptation. As scientific inquiry delves deeper into this field, the potential for therapeutic interventions aimed at modulating these epigenetic marks becomes increasingly viable. By tailoring treatments to the unique epigenetic profiles of individuals, clinicians can anticipate a future of personalized medicine, specifically attuned to mitigating the impacts of stress. This transformative understanding underscores the need for continued research and innovation in unraveling the mysteries of epigenetic regulation under stress.