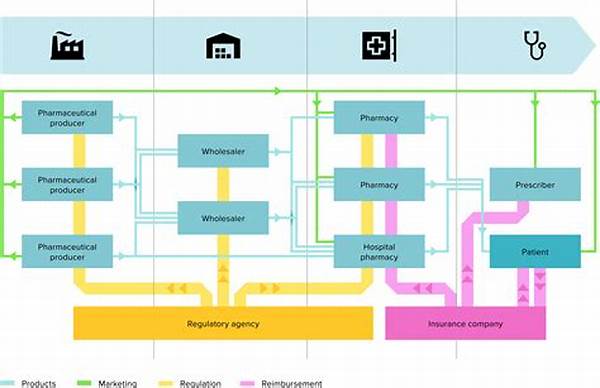
The regulation of prescription drugs is a cornerstone of public health policy worldwide. This intricate system ensures medications’ safety, efficacy, and quality before they reach consumers. Different countries have established agencies responsible for the oversight and regulation of prescription drugs, with frameworks designed to adapt to new scientific findings and evolving health challenges. These frameworks strike a critical balance between expedited access to necessary medications and rigorous assessment to avert potential risks. This article delves into the various aspects of prescription drug regulatory frameworks, offering insights into their structure and function.
Read Now : Risk-free Herb And Medicine Combination
Global Standards in Prescription Drug Regulation
Prescription drug regulatory frameworks are underpinned by global standards which ensure consistency and safety in drug manufacturing and distribution. The World Health Organization (WHO) sets international guidelines, urging countries to adopt robust regulatory mechanisms to protect public health. These frameworks often include processes for drug evaluation, manufacturing inspections, and post-market surveillance. By aligning with international standards, countries can ensure their regulatory frameworks are equipped to handle the complexities of modern pharmaceuticals and cross-border health challenges.
The frameworks vary by country, tailored to specific healthcare ecosystems. For instance, the United States’ FDA and the European Medicines Agency (EMA) have comprehensive regulatory processes that include thorough clinical trial evaluations and stringent approval protocols. These elements are essential to uphold the safety and effectiveness of drugs available to the public. Moreover, prescription drug regulatory frameworks are designed to be adaptable, accommodating scientific advancements and new therapeutic approaches.
Technological advancements and globalization present new challenges and opportunities for regulators. Prescription drug regulatory frameworks are evolving, incorporating digital technology to streamline assessment processes and enhance monitoring capabilities. International collaborations and harmonization efforts are becoming increasingly important in addressing global health crises and ensuring equitable access to essential medicines worldwide.
Key Components of Prescription Drug Regulatory Frameworks
1. Clinical Trial Evaluations: Prescription drug regulatory frameworks typically emphasize the importance of clinical trials, which serve as a vital component in assessing a drug’s safety and efficacy before approval.
2. Approval Protocols: Stringent approval protocols are a hallmark of effective prescription drug regulatory frameworks, ensuring all medications meet specific standards before entering the market.
3. Post-Market Surveillance: Continuous monitoring of approved medications is crucial, with prescription drug regulatory frameworks incorporating robust surveillance systems to identify and mitigate risks.
4. International Harmonization: Global cooperation is vital for addressing disparities in regulatory systems, with frameworks working towards harmonizing regulations to ensure consistency.
5. Adaptive Regulations: With rapid scientific developments, prescription drug regulatory frameworks must be adaptable, integrating new evidence and technologies to remain effective.
Challenges in Prescription Drug Regulatory Frameworks
Prescription drug regulatory frameworks face a myriad of challenges in the modern healthcare landscape. One significant issue is the need for rapid response mechanisms to address public health emergencies. During crises such as pandemics, the demand for swift drug approvals intensifies, posing challenges in maintaining rigorous safety standards. Balancing speed and accuracy in drug approvals is a delicate task, yet crucial to ensuring public trust and safety.
The rise of personalized medicine and biotech innovations also poses challenges to traditional regulatory frameworks. New therapeutic modalities, such as gene therapies, require specialized evaluation processes and expertise, straining existing regulatory capabilities. As prescription drug regulatory frameworks evolve, they must incorporate new scientific insights to effectively regulate these advanced treatments without compromising safety standards.
Additionally, the globalization of the pharmaceutical industry necessitates greater international cooperation. Prescription drug regulatory frameworks must adapt to handle cross-border issues, such as counterfeit drugs and varying regulatory standards across different regions. Strengthening global partnerships and fostering information sharing are essential to addressing these challenges and ensuring that all countries maintain high safety and quality standards for prescription drugs.
Informal Insights on Drug Regulations
When breaking down the sometimes bewildering world of prescription drug regulatory frameworks, it helps to have a straight-forward guide. Lost in the jargon? No worries, these 10 simple points will get you up to speed in the slang style. Think of these as the “prescription drug regulatory frameworks for dummies” cheat sheet.
1. The List of Essentials: Every country has their essentials on decks, like prescription drug regulatory frameworks to keep tabs on medicine safety.
2. Trial and Error: Drug regulations are all about testing. It’s not just tossing a pill out there. Rigorous trials, people!
3. Watchdog Duty: After approval, there’s a watchful eye over drugs. Yep, prescription drug regulatory frameworks make sure it’s not ‘set it and forget it.’
Read Now : Customized Medical Care Via Genomics
4. Emergency Button: Sometimes, it’s rush hour in drug approval town. Emergencies mean these frameworks have a FastPass option.
5. Mixing It Up: Innovations can throw some regulation curveballs. Prescription drug regulatory frameworks gotta keep their cool with new therapies.
6. Cross-Border Connections: Countries geek out collaboratively on regulations to shuffle out fake meds. It’s like a big pharma friendship circle.
7. Flexibility is Key: Prescription drug regulatory frameworks that are stiff as a board? Nah, they need ninja-like adaptability.
8. Playing it Safe: No skimping on safety – frameworks have a ‘safety-first’ mantra for approval.
9. Global Tune-Up: Different countries, but similar playlists. Frameworks strive for a worldwide harmony.
10. Trust in the System: With prescription drug regulatory frameworks, it’s all about trust. If they’re rock-solid, we feel safe popping pills.
Evaluation of the Regulatory Process
In the complex realm of prescription drug regulatory frameworks, the evaluation of the regulatory process plays a critical role. The evaluation process is meticulously conducted to ascertain whether a drug meets established criteria for safety, efficacy, and quality. This evaluation involves multiple stages, including preclinical studies, clinical trials, and reviews by regulatory experts. By adhering to rigorous evaluation protocols, prescription drug regulatory frameworks ensure that only those drugs that meet stringent standards are approved for consumer use.
The significance of this evaluation process cannot be overstated, as it serves as the foundation for maintaining public trust in the healthcare system. Errors or lapses in this process can lead to detrimental effects on public health, emphasizing the need for transparency and consistency in evaluation. Furthermore, prescription drug regulatory frameworks must periodically review and update their evaluation criteria to incorporate new scientific insights and address emerging health challenges. By doing so, the frameworks remain relevant and capable of safeguarding the health and well-being of the global population.
Adaptation and Evolution of Regulatory Frameworks
As the landscape of pharmaceuticals continues to evolve, so too must the prescription drug regulatory frameworks that govern it. This evolution is driven by several factors, including advancements in medical research, the globalization of drug markets, and the growing complexity of new therapeutic approaches. For regulators, the challenge lies in adapting existing frameworks to accommodate these developments without compromising the safety and efficacy standards that are fundamental to public health protection.
In response to these challenges, regulatory frameworks increasingly rely on innovative approaches such as real-world evidence and adaptive trial designs to expedite drug approvals while ensuring comprehensive evaluations. Prescription drug regulatory frameworks are also fostering greater international collaboration, sharing information and harmonizing practices to enhance regulatory efficiencies and address global health crises more effectively. By embracing these changes, regulators can create a more agile and responsive system that keeps pace with the dynamic nature of modern pharmaceuticals.
Summary of Prescription Drug Regulatory Frameworks
Prescription drug regulatory frameworks represent a pivotal component of public health strategies across the globe, functioning to protect consumers from potential risks associated with medication use. By instituting stringent processes for evaluation and approval, these frameworks ensure that drugs distributed to the public are safe, effective, and high quality. The critical role of these frameworks extends to post-market surveillance, maintaining oversight even after a drug has been released to consumers.
However, challenges persist in the realm of prescription drug regulatory frameworks, with emerging therapies and global health threats necessitating continual adaptation and innovation. Through international collaboration and the adoption of advanced evaluation methodologies, these frameworks can enhance their capacity to address such issues and secure the safety of pharmaceuticals. Ultimately, the success of prescription drug regulatory frameworks hinges on their ability to balance the imperative of rapid drug access with the uncompromising need for thorough safety assessments.