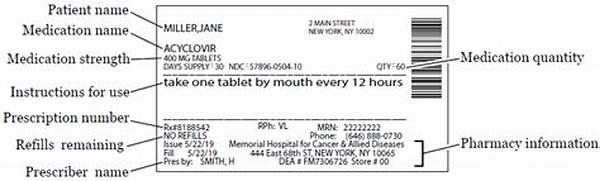
Importance of Prescription Medication Labeling Requirements
In the realm of pharmaceuticals, prescription medication labeling requirements play a pivotal role in ensuring patient safety and effective communication of critical information. These requirements are essential for healthcare providers, pharmacists, and patients alike. Labels serve as the cornerstone for conveying dosage instructions, potential side effects, contraindications, and storage guidelines. Without the stringent adherence to prescription medication labeling requirements, the risk of medication errors and adverse drug reactions could substantially increase.
Read Now : Harmonious Living With Nature
A crucial aspect of prescription medication labeling requirements is the standardization and clarity of the information presented. The labels must meet specific government regulations, ensuring they are legible, user-friendly, and comprehensible to all individuals involved in medication administration and consumption. This uniformity aids in minimizing confusion and promoting medication adherence, ultimately enhancing patient outcomes.
Health authorities, such as the FDA in the United States, have established comprehensive guidelines for prescription medication labeling requirements. These guidelines mandate the inclusion of both proprietary and generic drug names, precise dosage strengths, and clear instructions for use. Additionally, prescription medication labeling requirements necessitate a detailed account of any potential adverse reactions, drug interactions, and warning labels to safeguard against misuse. Thus, adhering to these standards is imperative for ensuring the safe and effective use of prescribed medications.
Key Elements of Prescription Medication Labeling Requirements
1. Label Clarity: Prescription medication labeling requirements demand that all labels must be clear and legible to avoid any misunderstanding or misadministration of the drug.
2. Standardized Format: Consistency in the labeling format is crucial, as per prescription medication labeling requirements, to facilitate easy comprehension and universal understanding.
3. Comprehensive Information: Labels must include all necessary details, such as active ingredients, usage instructions, and potential side effects, in adherence to prescription medication labeling requirements.
4. Regulatory Compliance: Prescription medication labeling requirements necessitate strict adherence to regulatory guidelines established by relevant health authorities.
5. Patient Safety: Ensuring patient safety is the primacy of prescription medication labeling requirements, achieved by providing thorough and unambiguous labeling instructions.
Regulatory Framework Surrounding Prescription Medication Labeling Requirements
Prescription medication labeling requirements are governed by a strict regulatory framework designed to maintain high standards of patient safety and drug efficacy. Regulatory agencies such as the FDA in the United States outline these stringent standards, which manufacturers must adhere to meticulously. These requirements ensure that the information presented on prescription medication labels is accurate, precise, and conducive to informed patient care.
The framework governing prescription medication labeling requirements encompasses various elements, including the inclusion of therapeutic indications, contraindications, and pharmacokinetics. This comprehensive approach ensures that healthcare providers have access to ample information when prescribing and dispensing medications, thus safeguarding against potential medication errors. Additionally, by mandating the disclosure of any off-label uses, regulatory bodies reinforce transparency and accountability within the pharmaceutical industry.
The enforcement of these prescription medication labeling requirements is not only a legal obligation but also a moral responsibility towards ensuring the safety and well-being of patients. Manufacturers must cooperate fully with regulatory bodies to ensure that any new findings, warnings, or changes in drug information are promptly reflected in the labeling. This collaboration is vital in maintaining the integrity of the healthcare system and upholding public trust in prescription medications.
Informal Insight: Decoding Prescription Medication Labeling Requirements
1. Labels gotta be on point—prescription medication labeling requirements aren’t kid stuff.
2. Clarity is king—if the text’s blurry, someone’s gonna mess up!
3. Same-same for all—standardized formats make labels a snap to read.
4. No secrets—spill the beans on side effects and more; requirements demand it.
Read Now : Cancer Treatment Based On Genomic Changes
5. Big brother (agencies) is watching—keep those labels in check.
6. Patient safety, always—it’s the heart of prescription medication labeling requirements.
7. Got all the info? Label needs full disclosure as per requirements.
8. Rules rule—follow prescription medication labeling requirements to a tee.
9. Fried circuits? Use regulated guidelines to avoid ’em.
10. Keep clear, keep safe—requirements ensure this duo.
Prescription Medication Labeling Requirements: An Examination
In examining the prescription medication labeling requirements, it is pivotal to appreciate their comprehensive nature in achieving safe medication practices. These requirements serve as a benchmark for standardizing the pharmaceutical labeling process, facilitating an environment devoid of ambiguity and misunderstanding. Labels crafted under these stringent standards enable healthcare providers, pharmacists, and patients to engage with medication more efficiently and safely.
Detailed prescription medication labeling requirements include the incorporation of universal symbols and language that are accessible and interpretable by all users. The seamless integration of such uniformity in labels is significant in promoting medication adherence and reducing the incidence of medication errors. By mandating the inclusion of crucial information, ranging from dosage and administration instructions to potential side effects and interactions, prescription medication labeling requirements ensure that all stakeholders are adequately informed.
Focused Observations on Prescription Medication Labeling Requirements
Prescription medication labeling requirements are an essential facet of pharmacovigilance and patient care. The intricate nature of these requirements underpins the need for precision and accuracy in the dissemination of drug-related information. Each label must be a testament to regulatory compliance, reflecting a standardization that transcends geographical boundaries, ensuring worldwide drug safety.
In practice, these requirements demand that labels exhibit exhaustive details, including pharmacological properties, therapeutic utility, and any warnings or contraindications. The rigidity of these stipulations is a testament to their crucial role in minimizing adverse drug events and fostering a culture of informed healthcare decisions. Labels, therefore, become more than mere appendages to drug packaging; they emerge as vital components of the therapeutic process in ensuring optimal patient care.
Synopsis on the Role of Prescription Medication Labeling Requirements
In summary, prescription medication labeling requirements represent a critical component of the pharmaceutical landscape, pivotal in ensuring patient safety and effective medication management. These requirements are meticulously crafted to encapsulate a range of elements, including drug identity, dosage instructions, and potential warnings, all aimed at safeguarding against medication errors. The clarity and precision mandated by these requirements underscore their essential role in fostering transparency in drug administration.
Through a collaborative approach with regulatory bodies, manufacturers are tasked with adhering to these standards, ensuring that prescription medication labeling requirements are consistently met. This cooperation ensures that any new developments, be they modifications in drug indications or emerging side effect profiles, are accurately reflected in the labeling. In doing so, prescription medication labeling requirements continue to uphold the trust bestowed by healthcare providers and patients in the safety and efficacy of medications.