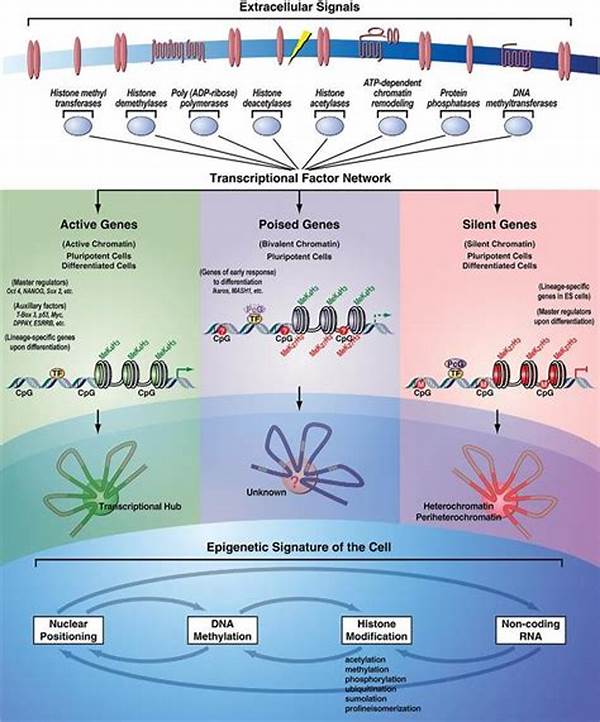
Understanding Tissue-Specific Epigenetic Mechanisms
Tissue-specific epigenetic mechanisms play a pivotal role in the regulation of gene expression across different cell types. These mechanisms enable cells with the same genetic information to execute distinct functions, thereby contributing to the complexity of multicellular organisms. Epigenetic modifications, such as DNA methylation, histone modification, and non-coding RNA action, are central to this regulatory framework. By facilitating or inhibiting the transcription of certain genes, these modifications allow tissues to develop unique phenotypic traits essential for specialized functions.
Read Now : Relaxation Techniques For Anxiety Disorders
In the context of developmental biology, tissue-specific epigenetic mechanisms are crucial for the differentiation process, guiding stem cells to develop into specific cell types. This differentiation defines the functional identity of cells, thereby ensuring the proper development and functioning of tissues and organs. For instance, while liver cells and brain cells originate from the same genetic blueprint, epigenetic modifications guide their divergent developmental pathways to fulfill specialized roles. Thus, understanding these mechanisms can illuminate the pathogenesis of various diseases, including cancer, where dysregulation of epigenetic patterns is frequently observed.
Moreover, tissue-specific epigenetic mechanisms have significant implications in regenerative medicine and therapeutic interventions. By manipulating these mechanisms, scientists hope to reprogram differentiated cells to a pluripotent state or to restore normal function in diseased tissues. As research in this field continues to advance, the potential to harness these mechanisms for novel medical treatments becomes increasingly promising. Consequently, elucidating the intricacies of tissue-specific epigenetic mechanisms remains a critical area of investigation in the life sciences.
Factors Influencing Tissue-Specific Epigenetic Mechanisms
1. Genetic predispositions influence how tissue-specific epigenetic mechanisms are established, contributing to individual variability in gene expression patterns.
2. Environmental factors play a significant role in modulating tissue-specific epigenetic mechanisms, as external stimuli can induce epigenetic changes.
3. Diet and nutrition impact tissue-specific epigenetic mechanisms by affecting the availability of methyl groups necessary for DNA methylation processes.
4. Cellular signaling pathways interact with tissue-specific epigenetic mechanisms, facilitating the translation of external signals into gene expression changes.
5. Age-related changes can lead to alterations in tissue-specific epigenetic mechanisms, affecting tissue function and homeostasis over time.
Advances in Research on Tissue-Specific Epigenetic Mechanisms
In recent years, substantial progress has been made in the field of tissue-specific epigenetic mechanisms, fueled by advancements in genomic technologies. Techniques such as chromatin immunoprecipitation sequencing (ChIP-seq), RNA sequencing (RNA-seq), and single-cell epigenomics have enabled researchers to map epigenetic modifications with unprecedented resolution and precision. These tools provide insights into the specific epigenetic landscapes of various tissues, elucidating how these modifications regulate tissue identity and function.
The integration of multi-omics approaches has furthered our understanding of tissue-specific epigenetic mechanisms. By combining genomics, transcriptomics, proteomics, and metabolomics data, researchers can construct comprehensive models that reveal complex interactions between different layers of cellular regulation. Such integrative analyses uncover the dynamic nature of epigenetic modifications and their responsiveness to physiological changes or pathological conditions. Ultimately, these insights have significant implications for disease diagnosis and the development of targeted therapeutic strategies that leverage the specificity of tissue-specific epigenetic mechanisms.
Furthermore, novel computational tools and machine learning methods have enhanced the analysis of large-scale epigenomic datasets, allowing for the identification of epigenetic signatures associated with specific tissues or conditions. These advancements promise to accelerate the pace of discovery in this field, leading to an improved understanding of the fundamental biological processes governed by tissue-specific epigenetic mechanisms. As research continues to advance, the capacity to manipulate these mechanisms for therapeutic purposes holds great promise for precision medicine and personalized healthcare approaches.
Tissue-Specific Epigenetic Mechanisms: A Slang Analysis
Yo, when it comes to tissue-specific epigenetic mechanisms, they’re like the real MVPs of gene regulation, switching things up in different cells. Picture this: cells with the same DNA, but thanks to epigenetics, they’re doing their own thing, living their best lives.
Think of tissue-specific epigenetic mechanisms as DNA’s personal stylist, dressing genes up or down depending on what the tissue needs. They’re like the DJ at a party, hyping up some genes and keeping others on mute.
Now, these tissue-specific epigenetic mechanisms don’t work in isolation, they’re vibing with signals from the environment – food, stress, you name it. It’s like a remix of life’s external beats translating into internal gene tunes.
Read Now : Evidence-based Medical Practice Guidelines
Scientists are all about cracking the code of these mechanisms. Why? Because if we can hack those tissue-specific epigenetic mechanisms, we might just rewrite the script on diseases, turning bad news into good vibes.
And when it comes to aging, these mechanisms are like the secret sauce. They keep tissues functioning right over time, but if they wobble, that’s when age starts to show its cards.
In the healthcare scene, the potential of tissue-specific epigenetic mechanisms is massive. Imagine tweaking them just right; suddenly, we’re talking regenerative medicine hitting the mainstream, making waves in healing vibes.
Implications for Health and Disease
Interplay of Epigenetics and Disease
Understanding the role of tissue-specific epigenetic mechanisms in health and disease has become a major focus in biomedical research. Aberrations in these mechanisms are frequently associated with pathologies, providing potential biomarkers and therapeutic targets. For instance, cancer cells often exhibit disrupted epigenetic patterns that lead to uncontrolled proliferation, indicating that restoring normal epigenetic states could inhibit tumor growth. Similarly, autoimmune diseases, neurological disorders, and metabolic syndromes have been linked to epigenetic dysregulation, highlighting the widespread impact of these mechanisms on human health.
Moreover, the specificity of tissue-specific epigenetic changes offers promising avenues for precision medicine. By designing interventions that target aberrant epigenetic modifications within affected tissues, therapies can be more effective with reduced systemic side effects. Epigenetic drugs, including methylation inhibitors and histone deacetylase inhibitors, are under investigation, aiming to modulate these modifications to restore normal cellular functions. As research progresses, the potential of targeted epigenetic therapies continues to emerge, promising innovative treatments for previously intractable diseases.
Future Directions in Epigenetic Research
The burgeoning field of tissue-specific epigenetic mechanisms presents numerous avenues for future exploration, particularly in understanding their contribution to complex phenotypes and disease susceptibility. As technology advances, researchers aim to map comprehensive epigenomes across diverse cell types and conditions to identify critical modifications that drive pathological states. Additionally, exploring the interactions between genetic predispositions and epigenetic changes is crucial for elucidating the multifaceted nature of disease etiology.
Developing personalized epigenetic profiles could revolutionize medical practice, enabling clinicians to predict disease risk and tailor interventions based on individual epigenetic landscapes. Furthermore, elucidating the dynamic nature of tissue-specific epigenetic modifications in response to environmental stimuli and lifestyle factors can offer insights into prevention strategies. Public health initiatives focusing on modifiable risk factors, such as diet and exercise, could leverage this knowledge to promote healthy epigenomes and mitigate disease onset.
Summary of Tissue-Specific Epigenetic Mechanisms
Insights and Significance
In summary, tissue-specific epigenetic mechanisms are fundamental to the regulation of gene expression, facilitating the differentiation and functioning of diverse cell types. These mechanisms, including DNA methylation, histone modification, and the action of non-coding RNAs, enable cells to acquire tissue-specific identities despite sharing a common genome. Their role is paramount in development, enabling stem cells to differentiate into various tissues.
The significance of tissue-specific epigenetic mechanisms extends to understanding disease processes, as disruptions in these regulatory pathways are implicated in numerous pathologies. From cancer to metabolic disorders, the aberration of epigenetic patterns underscores the importance of maintaining epigenetic homeostasis for optimal health. Consequently, ongoing research aims to elucidate the interactions between these mechanisms and genetic or environmental factors, seeking novel insights into disease prevention and treatment.
Ultimately, the field holds tremendous potential for advancing precision medicine, as the specificity of tissue-specific epigenetic modifications allows for targeted interventions with minimal off-target effects. By leveraging our growing understanding of these mechanisms, the development of epigenetic therapies offers promising prospects for the management of complex diseases, underscoring the critical role of epigenetics in shaping future healthcare paradigms.