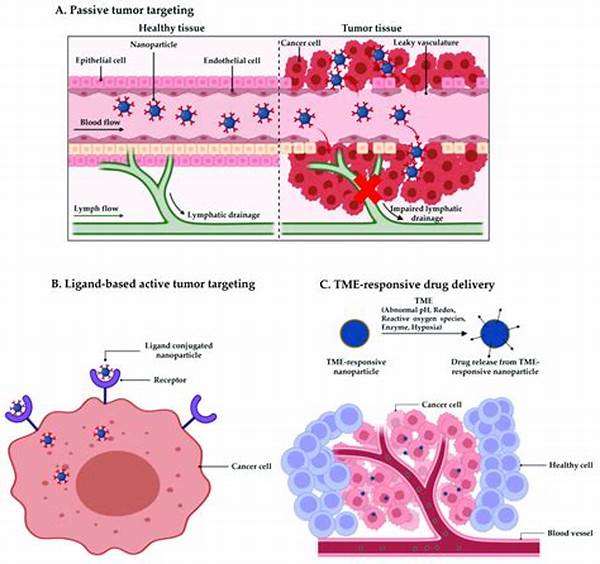
Introduction to Tumor-Targeting Nanocarrier Platforms
In recent years, the advent of nanotechnology has revolutionized the landscape of cancer treatment, with tumor-targeting nanocarrier platforms standing at the forefront of this innovation. These platforms are engineered with precision, aiming to deliver therapeutic agents directly to cancerous cells, thereby minimizing damage to healthy tissues. The strategic design and tailoring of these nanocarriers are pivotal in enhancing their targeting specificity and efficiency. Through a marriage of scientific ingenuity and technological advancement, tumor-targeting nanocarrier platforms represent a significant leap towards personalized medicine. Their ability to pinpoint and directly combat malignant cells is not only a testament to progress in medical science but also offers hope in reducing the adverse side effects associated with traditional cancer treatments.
Read Now : Chronic Drug Intake Consequences
The core advantage of tumor-targeting nanocarrier platforms lies in their unique architectural design, which can be customized to carry drugs, genes, or imaging agents. This modularity facilitates versatility, allowing for broad applications across various types of cancers. Additionally, these platforms are often engineered to respond to specific triggers within the tumor microenvironment, such as pH levels or enzymatic activities, ensuring that their payload is released at the most opportune moment. Such controlled release mechanisms are integral in maximizing therapeutic efficacy while minimizing systemic toxicity.
Furthermore, continued research and development in the field of tumor-targeting nanocarrier platforms are anticipated to enhance their efficacy and safety profiles. With ongoing advancements in biomaterials and nanocarrier designs, the future of these platforms looks promising. As the field progresses, collaborations between multidisciplinary teams of researchers, clinicians, and engineers will be crucial in overcoming current limitations and accelerating the clinical translation of these promising nanotechnology-based therapeutic strategies.
Strategic Advancements in Tumor-Targeting Nanocarrier Platforms
The development of tumor-targeting nanocarrier platforms has seen significant advancements. Researchers focus on optimizing the payload capacity of these carriers to enhance their efficacy. Additionally, efforts are being made to improve the surface functionalization of nanocarriers, enabling better interaction with cancer cell receptors. Tumor-targeting nanocarrier platforms are leading the frontier of modern oncology.
Another strategic focus is the enhancement of nanocarrier biocompatibility. Researchers are investigating novel biomaterials that mimic natural cellular components to reduce immune system recognition. This approach increases circulation time, resulting in better accumulation at tumor sites. Such improvements in tumor-targeting nanocarrier platforms represent a shift towards more effective and less intrusive cancer treatment modalities.
The integration of imaging functions into tumor-targeting nanocarrier platforms provides dual benefits of treatment and diagnostics. Known as theranostic platforms, these innovations enable real-time monitoring of drug delivery and treatment response. This dual functionality not only enhances patient outcomes but also provides critical data that can refine and improve existing therapeutic protocols.
Efforts are also directed at overcoming the biological barriers that impede drug delivery efficacy. Tumor-targeting nanocarrier platforms are being engineered to navigate these obstacles, such as dense stromal tissue or an acidic tumor microenvironment. By addressing these barriers, nanocarriers enhance drug penetration and efficacy, demonstrating their potential in improving cancer treatment outcomes.
Research in tumor-targeting nanocarrier platforms extends to overcoming multidrug resistance, a prevalent issue in cancer therapy. By delivering drugs in nanoparticles that evade typical resistance mechanisms, these platforms offer renewed hope for effective treatment paths for resistant cancer types. Such innovation underscores their transformative potential in oncology.
Challenges and Opportunities in Tumor-Targeting Nanocarrier Platforms
While the potential of tumor-targeting nanocarrier platforms is vast, their implementation is not without challenges. One of the significant hurdles involves ensuring the stability of these nanocarriers in the bloodstream. Premature degradation can lead to insufficient drug delivery to the target site, thereby compromising treatment efficacy. Stability improvements necessitate advancements in nanocarrier material science, requiring multidisciplinary research collaboration.
Another key challenge lies in perfecting the targeting specificity of tumor-targeting nanocarrier platforms. Non-specific interactions with healthy cells pose a risk of off-target effects, which may lead to unintended side effects. To overcome this, researchers are focusing on improving the selectivity of ligands attached to the nanocarriers, which could significantly enhance the homing abilities of these platforms to cancerous tissues.
Despite these challenges, the opportunities presented by tumor-targeting nanocarrier platforms remain promising. The potential to combine various therapeutic agents within a single nanocarrier opens avenues for combination therapies, which have shown great promise in improving treatment efficacy. As research continues to advance, the ability to personalize these platforms according to patient-specific cancer profiles suggests a future of highly tailored cancer treatments, optimizing therapeutic outcomes with minimal adverse effects.
Innovations in Tumor-Targeting Nanocarrier Platforms
1. Payload Integration: Tumor-targeting nanocarrier platforms are designed to carry multiple therapeutic agents simultaneously, enhancing combinational cancer therapy outcomes.
2. Surface Functionalization: Enhanced surface properties allow tumor-targeting nanocarrier platforms to interact specifically with tumor cell receptors, improving targeting accuracy.
3. Biocompatibility Enhancements: Innovations focus on minimizing immune system recognition of tumor-targeting nanocarrier platforms, resulting in longer circulation times in vivo.
4. Overcoming Biological Barriers: Engineering solutions aim to enhance the ability of tumor-targeting nanocarrier platforms to penetrate cellular barriers, boosting drug delivery efficacy.
5. Theranostic Capabilities: Tumor-targeting nanocarrier platforms offer dual functionalities, combining therapy and diagnostics for improved monitoring and treatment adaptability in oncology.
6. Reduced Multi-Drug Resistance: By evading traditional resistance mechanisms, tumor-targeting nanocarrier platforms present effective solutions against resistant cancer forms.
Read Now : Effective Herbal Medicine Pairings
7. Stimuli-Responsive Systems: Unique designs enable tumor-targeting nanocarrier platforms to release therapeutic agents in response to specific tumoral triggers, enhancing precision.
8. Improved Stability: Innovations focus on ensuring that tumor-targeting nanocarrier platforms maintain structural integrity, ensuring effective delivery to target sites.
9. Personalized Medicine Potential: Tumor-targeting nanocarrier platforms enable customization for patient-specific treatment, promoting personalized and effective cancer therapies.
10. Cross-Disciplinary Collaborations: Continued advances in tumor-targeting nanocarrier platforms rely on collaborative efforts across multiple scientific disciplines for realistic clinical applications.
Materials and Design of Tumor-Targeting Nanocarrier Platforms
The field of tumor-targeting nanocarrier platforms has undergone transformative advancements, notably in the aspects of materials and design. These platforms generally consist of organic and inorganic materials that are biocompatible and capable of bearing therapeutic loads without eliciting adverse immune reactions. Polymers, lipids, and metallic nanoparticles are commonly used, each offering unique advantages in terms of stability and functionalization potential.
Polymeric carriers often facilitate versatile design modifications, allowing researchers to tweak external functionalities for specific targeting purposes. Lipid-based nanocarriers, on the other hand, mimic natural cell membranes, enhancing biocompatibility and reducing immunogenicity. Inorganic materials like gold or silica nanoparticles offer structural stability and can be readily functionalized with targeting ligands. The choice of material in tumor-targeting nanocarrier platforms is crucial, impacting their overall efficacy, safety, and precision in targeting tumor sites.
The design of these nanocarriers is also integral to their function. Advanced surface engineering techniques are employed to enhance their targeting capabilities, often by decorating them with ligands or antibodies that recognize tumor-specific markers. The size, shape, and surface charge of these nanocarriers are meticulously optimized to ensure prolonged circulation time and precise accumulation within tumor tissues. Such meticulous design considerations underscore the intricate balance between functionality and safety in the development of tumor-targeting nanocarrier platforms.
Regulatory Aspects and Clinical Challenges of Tumor-Targeting Nanocarrier Platforms
The path from conceptual design to clinical application of tumor-targeting nanocarrier platforms involves navigating complex regulatory landscapes and addressing numerous clinical challenges. Regulatory bodies require rigorous testing to ensure these platforms’ safety, efficacy, and quality before they can be approved for human use. Comprehensive preclinical studies are necessary for revealing potential toxicities and biological interactions, forming the basis for subsequent clinical trials.
Current clinical trials involving tumor-targeting nanocarrier platforms face challenges including variability in human response and the complexity of biological systems. Ensuring consistent and reliable production scale is another significant hurdle, as small-scale laboratory results are not always replicable in industrial settings. Moreover, these platforms must demonstrate a clear advantage over existing therapies to gain regulatory approval, presenting an ongoing challenge for researchers and developers.
Despite these challenges, the continued refinement of tumor-targeting nanocarrier platforms portends a promising future. Regulatory frameworks are gradually adapting to accommodate the unique features of nanotechnology-based therapeutics, which in turn supports ongoing research and development in this field. As regulatory bodies and scientific communities work in tandem, the goal remains to bridge the gap between laboratory innovation and clinical implementation, thus broadening the horizon for effective cancer treatments.
Summary of Tumor-Targeting Nanocarrier Platforms
In summary, tumor-targeting nanocarrier platforms are poised to amend the paradigm of cancer therapeutics. By focusing on precision delivery and minimal side effects, these platforms align with the overarching goal of personalized medicine. Their modular design allows them to be tailored for various therapeutic agents, offering an adaptable and robust option for combating cancer. As the understanding of tumor biology deepens, these platforms are being further optimized to interact specifically with tumoral environments.
The road to implementation is fraught with challenges, primarily revolving around stability, targeting specificity, and clinical translation. Nonetheless, ongoing research endeavors aim to enhance each aspect of tumor-targeting nanocarrier platforms painstakingly. The successful integration of imaging functionalities within these platforms underscores their potential in revolutionizing both diagnostic and therapeutic processes.
Interdisciplinary collaborations and adaptive regulatory frameworks are pivotal to overcoming barriers related to clinical testing and approval. As innovation continues to flourish, tumor-targeting nanocarrier platforms will likely become an integral part of modern oncology, paving a new path towards safer and more efficient cancer treatment methodologies. Their potential extends beyond mere therapeutics, providing critical data and insights that are invaluable in shaping the future of cancer management.